Document Type : Review Article
Authors
1 Department of Microbiology Federal University of Technology Owerri, Nigeria
2 Department of BioTechnology Federal University of Technology Owerri, Nigeria
3 Department of Chemical Sciences, Federal University Wukari, Taraba State, Nigeria
4 Department of Anatomy, Ebonyi State University, Abakaliki, Nigeria
Abstract
Microalgae have garnered significant attention as a promising source for biodiesel production due to their rapid growth, high lipid content, and minimal resource requirements. This study delves into the forefront of microalgal biofuel research by investigating emerging co-cultivation strategies aimed at optimizing biomass production for biodiesel synthesis. Through a nuanced exploration of diverse microalgal strains, this work pioneers innovative co-cultivation techniques designed to enhance synergies between different species, thus maximizing overall productivity. Microalgae are excellent options for the production of biodiesel since they have high lipid content and grow quickly. Nonetheless, difficulties in maximizing lipid and biomass production have led the technological advancement microalgal productivity using photobioreactors, closed systems and monitoring and genetic Engineering The article delves into the impact of Co-cultivation on microalgal growth such as improved Biomass production, enhanced lipid content and quality and nutrient utilization and recycling.
Graphical Abstract
Keywords
Main Subjects
Introduction
The most promising and ecologically benign alternative among the competitors is microalgal biodiesel. Due to their high lipid content, adaptability to a wide range of conditions, and quick growth, microalgae are highly valued. In the 1970s and 1980s, pioneering studies focused on identifying suitable microalgal strains and optimizing cultivation techniques. However, early research faced challenges related to economic viability and scalability. During the 1990s and early 2000s, there was a notable shift toward enhancing lipid productivity in microalgae. Researchers aimed to identify and genetically modify strains with higher lipid accumulation, addressing one of the primary limitations of microalgal biodiesel production. Improved understanding of microalgal physiology, lipid metabolism, and stress-induced lipid production mechanisms contributed to advancements in strain selection and genetic engineering [1]. The 2010s marked a period of intensified research efforts with a focus on technological innovations and process optimization. In recent years, researchers have increasingly explored co-cultivation strategies as a means to address challenges related to nutrient utilization, competition, and environmental stress. Studies on microalgal consortia and symbiotic relationships have gained prominence, offering insights into how co-cultivation can enhance biomass productivity and lipid yields. Microalgae biomass refers to the collective mass of microalgae cells, which are microscopic, photosynthetic organisms that convert sunlight into energy through photosynthesis. Microalgae are unicellular or simple multicellular algae that can be found in various aquatic environments, including freshwater and marine systems. They play a crucial role in the ecosystem by producing oxygen and serving as a primary food source for various aquatic organisms. The biomass of microalgae is of interest in various fields due to its potential applications in biotechnology, bioenergy, and environmental management [2]. Microalgae are rich in bioactive compounds, including proteins, lipids, pigments, and carbohydrates. The biomass can be utilized in the production of biofuels, nutraceuticals, pharmaceuticals, and other high-value products. Furthermore, microalgae are considered a promising feedstock for biofuel production, such as biodiesel and bioethanol. The high lipid content in certain microalgae species makes them particularly suitable for biofuel applications and is known for their ability to absorb and assimilate nutrients, heavy metals, and pollutants from water. This makes them useful in environmental remediation efforts, such as wastewater treatment and nutrient recovery. Some microalgae species are used as a nutritional supplement in aquaculture and animal feed industries. They provide essential nutrients and enhance the nutritional profile of the feed [3-4]. Biodiesel is a renewable and cleaner-burning alternative to conventional diesel fuel, typically produced from biological sources such as vegetable oils, animal fats, or recycled cooking oils. It is a type of biofuel that can be used in diesel engines with little to no modification. Biodiesel is typically produced through a chemical process called transesterification. In this process, triglycerides (fats and oils) are reacted with an alcohol (usually methanol or ethanol) in the presence of a catalyst to produce biodiesel and glycerol. In the pursuit of sustainable energy sources and environmental conservation, microalgae have emerged as a promising candidate for biofuel production, specifically biodiesel. Microalgae exhibit high growth rates, efficient photosynthetic capabilities, and the ability to accumulate lipids, making them a valuable feedstock for biodiesel. The study delves into the realm of emerging co-cultivation strategies for microalgal biomass and biodiesel production, highlighting the potential breakthroughs and advancements that could revolutionize the bioenergy landscape [5]. The process of making microalgal products involves numerous steps, including the creation and cultivation of the microalgal strains, the harvesting of the microalgal biomass from the culture media, and the subsequent thickening, dewatering, drying, and extraction of the desired product [6]. Large-scale energy usage and production costs of microalgal-based products are significantly impacted by efficient and economical harvesting and drying techniques. Microalgal biomass is a great source of triglycerides, vitamins, pigments, and vital amino acids. It can be utilized on its own or combined with other materials to provide nutritious feed and food for aquaculture [7].
Co-cultivation techniques can increase biomass and lipid productivity, enabling the commercialization of microalgal-based fuel [8].
Microalgae are eukaryotic, unicellular, and photosynthetic organisms that may be grown on non-arable land, effectively absorb carbon dioxide, and recover nutrients from wastewater. The co-cultivation of microalgae with other microorganisms presents a viable approach to augment biomass and lipid output, both of which are important for the biodiesel manufacture [9-10]. The co-cultivation of microalgae with other microorganisms presents a viable approach to augment biomass and lipid output, both of which are important for the manufacture of biodiesel. In co-cultivation, microalgae grow in symbiotic relationships with other heterotrophic microbes like fungi, bacteria, yeast, and other algae. Through this process, the microalgae productivity is increased through the exchange of nutrients and metabolites [11]. Commercialization of microalgal-based fuel can be aided by co-cultivating microalgae with other microbes to increase biomass and lipid output [12-14].
The aim of this study is to present a thorough analysis of co-cultivation techniques for the production of biodiesel and microalgal biomass. The study is embedded in the transformative paradigm it introduces to the field of microalgal biofuel production. This research explores innovative co-cultivation strategies that transcend conventional approaches, aiming to address critical challenges and unlock new opportunities in the sustainable production of microalgal biomass for biodiesel. The unique aspects of this work lie in its progressive methodologies, experimental designs, and outcomes, collectively contributing to the advancement of knowledge and practices in the realm of microalgal-based biofuel production.
The scope encompasses an overview of microalgae, in the manufacturing of drugs, and biofuels. An examination of the methods used to co-cultivate microalgae with other microorganisms, including fungi, bacteria, yeast, and other algae, to increase biomass and lipid productivity
Microalgae as a source of biodiesel
A wide variety of microscopic photosynthetic organisms known as microalgae have drawn a lot of interest recently as a possible source of biodiesel. Microalgae, in contrast to terrestrial oilseed crops, have special qualities that make them a great option for producing biodiesel in an environmentally friendly manner [15]. An overview of the main factors driving the increased interest in microalgae as a biodiesel source is given in this section:
High lipid content
At over 50% of their dry weight, microalgae are renowned for having extraordinarily high lipid content. Triglycerides, the main raw material used in the manufacturing of biodiesel, make up the majority of these lipids. The lipid content of microalgae is far higher than that of conventional oilseed crops like soybeans and palm, which makes them a desirable source for biofuel feedstock [16].
Rapid growth
Microalgae grow quickly, often doubling their biomass in a few hours. This fast growth rate makes it possible to harvest them frequently and to cultivate them continuously, providing a reliable and renewable source of feedstock for the biodiesel production [17].
Versatile cultivation
Microalgae can be grown in a variety of environments, such as open ponds, photobioreactors, and wastewater treatment facilities. They can also be grown on non-arable land, which lessens competition with food crops.
Minimal land and water requirements
Microalgae can be grown on non-arable land and do not need fresh water to grow. Some species can even be grown using saline or wastewater, which addresses concerns about the amount of land and water used in the production of biofuel [18].
Reduced carbon footprint
Microalgae-based biodiesel has the potential to reduce greenhouse gas emissions. Large-scale cultivation of microalgae can help mitigate carbon emissions because they absorb carbon dioxide during photosynthesis. Nutrient Recycling: Microalgae can thrive on a variety of nutrient sources, including nitrogen and phosphorus. Their cultivation can serve a dual purpose in wastewater treatment systems by removing nutrients and producing biofuel feedstock [19].
Biodiesel quality
Biodiesel made from microalgal lipids has qualities that are on par with or even better than traditional diesel fuels, and it satisfies the quality requirements imposed for conventional biodiesel (such as ASTM D6751) [20].
Versatility of biodiesel
Microalgal biodiesel does not require major modifications to be used in current diesel engines. It is regarded as a drop-in substitute for fossil fuel diesel [21]. Microalgae are a viable and ecologically friendly source of biodiesel due to their high lipid content, quick development, flexibility, and sustainability. However, to fully realize the potential of microalgae in the manufacture of biodiesel, a number of obstacles need to be overcome, such as cost-effectiveness, scalability, and culture process improvement. This section lays the groundwork for a thorough investigation of microalgal biodiesel, emphasizing its enormous potential in the shift to renewable and sustainable energy sources [22].
Microalgal characteristics and lipid production
The high lipid accumulation capacity, rapid growth rate, and high photosynthetic efficiency of microalgae make them a promising source of lipids for the generation of biodiesel. Under rare circumstances, the lipid content of microalgae can reach up to 80% of the dry weight of the cell as shown in fig 1 [23]. Typically, it ranges from 20-50%. The two types of microalgal lipids that are categorized based on their carbon number are very-long-chain fatty acids (VLCFAs) and long-chain fatty acids (LCFAs). Waxes, sterols, hydrocarbons, glycerolipids, and other lipid-like substances are produced by microalgae [24]. Microalgae create a lipid called glycerolipid, which is the most prevalent and well-known kind.
As lipids are essential for the construction of cellular membranes and are produced by microalgal cells, their synthesis of lipids go beyond energy storage [25]. Microalgae's biomass production and lipid accumulation are regulated by both environmental factors and nutrition availability. The amount and mode of delivery of essential nutrients, like carbon or nitrogen sources, have a major impact on cell division and fat formation.
An effective way to raise the productivity of microalgal lipid and lower the cost of producing microalgal biofuels is to optimize environmental parameters and regulate the environment to improve the synthesis of microalgal lipid. Environmental elements that can impact the lipid production of microalgae include temperature, light intensity, salt content, and iron concentration [26].
Co-Cultivation approaches
Mixotrophic cultivation
In the field of producing biodiesel and microalgal biomass, mixotrophic cultivation is a promising approach. Mixotrophy allows microalgae to flourish in the presence of both organic carbon sources and light energy, in contrast to standard autotrophic farming, where microalgae only rely on photosynthesis for energy and carbon acquisition [27]. Mixotrophic cultivation is a co-cultivation strategy used to produce biodiesel and microalgal biomass. This method uses both inorganic and organic carbon sources to promote the growth of microalgae. Mixotrophic cultivation has been demonstrated to boost microalgae's biomass and lipid output, making it a viable method for producing biodiesel [28]. Microalgae can effectively use carbon resources in soil and water, which makes them an essential component of the natural carbon cycle [29]. They have the capacity to reduce CO2 emissions and yield highly productive oil, which makes them a viable biodiesel source. The process of producing biodiesel from microalgae involves recycling and integrating carbon resources from the natural world. This can help achieve the long-term objectives of displacing petroleum use and lowering environmental pollution, which will make a substantial contribution to the global effort to achieve carbon neutrality [30]. Mixotrophic cultivation has been demonstrated to boost microalgae's biomass and lipid output, making it a viable method for producing biodiesel. This method uses both inorganic and organic carbon sources to promote the growth of microalgae. Compared to autotrophic cultivation, mixotrophic farming can boost microalgae biomass and lipid output by up to 50%. Producing biodiesel and microalgal biomass using mixotrophic cultivation is a potential strategy.
It has been demonstrated to raise the lipid productivity and biomass of microalgae, making it a viable method for producing biodiesel. Mixotrophic horticulture can play a major part in the worldwide effort to achieve carbon neutrality by helping to reduce environmental pollution and eventually replace petroleum consumption [31].
Heterotrophic cultivation
Another co-cultivation strategy for producing microalgal biomass and biodiesel is heterotrophic cultivation. With this method, organic carbon sources are used to support the growth of microalgae. Heterotrophic culture is a viable method for producing biodiesel because it has been demonstrated to boost the biomass and lipid productivity of microalgae [32]. Microalgae are a promising source of biodiesel since they can produce oil with high productivity, reduce CO2 emissions, and effectively use carbon resources found in soil and water. To produce biodiesel from microalgae, carbon resources from the natural environment must be reused and integrated. This can help achieve the long-term objectives of displacing petroleum use and lowering environmental pollution, which will make a significant contribution to the global effort toward carbon neutrality [33]. It has been demonstrated that heterotrophic culture increases the biomass and lipid productivity of microalgae, making it a viable method for producing biodiesel. With this method, organic carbon sources are used to support the growth of microalgae. When compared to autotrophic cultivation, the adoption of heterotrophic cultivation can enhance the biomass and lipid productivity of microalgae by up to ten times [34].
A viable method for producing biodiesel and microalgal biomass is heterotrophic culture. It has been demonstrated to raise the lipid productivity and biomass of microalgae, making it a viable method for producing biodiesel. Heterotrophic agriculture can play a major part in the global effort to achieve carbon neutrality by helping to reduce environmental pollution and replace petroleum consumption in the long run [35-37].
Symbiotic co-cultivation
Another method of co-cultivation for producing microalgal biomass and biodiesel is symbiotic co-cultivation. In this method, various heterotrophic microbes such bacteria, yeast, fungus, and other algae or microalgae are grown in symbiosis with the microalgae [38]. Microorganisms exchange nutrients and metabolites during symbiotic co-cultivation, which boosts output and makes microalgal-based fuel more commercially viable [39]. Co-cultivation has proven to be an effective method for utilizing lipids, carbohydrates, and proteins to transform microalgae biomass into various biofuels, such as biodiesel, biohydrogen, bioethanol, biomethanol, biobutanol, and biomethane [40-42]. With no sulphur or aromatic byproducts produced, biodiesel is a promising renewable energy source that can drastically lower emissions of unburned hydrocarbons and carbon monoxide. Triglycerides (TAGs), an organic component that can be utilized as a precursor for the synthesis of biodiesel, are produced by microalgae from CO2 and water [43]. It has been demonstrated that symbiotic co-cultivation increases the microalgae's cellular oil, lipid content, and biomass production [44-47]. For instance, improvements were seen in the lipid content, cellular oil, and microalgae biomass production in the co-culture of Chlorella and Aspergillus. Similar improvements in biomass yield, lipids, and saturated and unsaturated fatty acids that can be used to produce biodiesel were demonstrated by the Mucor circinelloides and C. vulgaris consortia [48].
However, the choice of microorganisms and how they interact with one another greatly affects how efficient this strategy is. A viable method for producing biodiesel and microalgal biomass is symbiotic co-cultivation. It has been demonstrated to increase the lipid content, cellular oil, and biomass yield of microalgae, making it a viable method for producing biodiesel [49]. Symbiotic co-cultivation can play a major part in the global effort to achieve carbon neutrality by helping to reduce environmental pollution and replace petroleum consumption in the long run [50].
Co-cultivation with other microorganisms
Because of their high lipid content and quick growth rates, single-celled photosynthetic microorganisms known as microalgae have become a promising source for the generation of biomass and biodiesel. Large-scale microalgal production is hampered by a number of issues, though, such as nutrient competition, contamination risk, and the significant energy expenditure needed to maintain ideal culture conditions [51]. Co-cultivation strategies have gained a lot of attention as a way to get around these problems and improve the overall productivity and efficiency of biomass and biodiesel production. These strategies involve the simultaneous cultivation of microalgae with other microorganisms like bacteria, fungi, or yeasts [52].
Co-cultivation techniques with other microorganisms have shown promise in resolving issues related to the production of biodiesel and microalgal biomass. Even if there are still many obstacles to be solved, the continuous research and development in this area has great promise for the production of sustainable biofuels and the reduction of environmental problems related to the use of conventional fossil fuels [53]. Future developments in co-cultivation techniques have the potential to make the biofuel sector more environmentally friendly and sustainable.
Bacterial Co-cultivation
To promote a symbiotic interaction between the two microorganisms, bacteria and microalgae are grown simultaneously in a shared habitat in a process known as "bacterial co-cultivation." In a mutualistic interaction, the bacteria and microalgae gain from one another's company [54]. Commensal relationships occur when one organism gains an advantage while the other is neither aided nor hindered. Some benefits of Bacterial Co-cultivation include:
Nutrient cycling
The improvement of nutrient cycling is one of the main advantages of bacterial co-cultivation. For example, nitrogen-fixing bacteria can transform atmospheric nitrogen into a form that microalgae can easily use, negating the requirement for additional nitrogen replenishment. This results in cost savings and more effective nutrient utilization.
Contaminant control
In co-cultivation systems, bacteria can serve as sentinels by displacing undesirable microorganisms, diseases, or algae that could contaminate the culture. This lower chance of contamination can greatly increase the microalgal culture's dependability and stability [55].
Improvement in biomass and lipid yields
Some bacteria can boost lipid production or encourage the growth of microalgae. Certain bacteria, for instance, emit compounds that encourage growth, while other bacteria benefit microalgae by eliminating harmful chemicals or lessening oxidative stress. Lipid yields and biomass are raised as a result [56].
CO2 utilization
Utilizing bacterial co-cultivation, carbon dioxide (CO2) emissions from industrial operations can be captured. During photosynthesis, microalgae absorb CO2, and their growth is stimulated by an atmosphere that is high in CO2. This method can generate useful biomass and biodiesel while reducing greenhouse gas emissions [57].
Fungal Co-cultivation
A new symbiotic strategy for increased biomass and biodiesel production is fungus co-cultivation. It entails growing fungi and microalgae together in a common habitat in order to foster a symbiotic relationship between the two microbes [58]. Microalgae and fungi may have a mutualistic connection in which both parties gain, or a commensal relationship in which one organism gains and the other does not. Some benefits of Fungal Co-cultivation are:
Nutrient recycling
Microalgae can more easily acquire nutrients when complex organic matter is broken down by fungi. They have the ability to break down organic materials and liberate nutrients from complicated molecules, which helps the co-cultivation system recycle nutrients like phosphorus and nitrogen.
Contaminant suppression
By producing antimicrobial chemicals and enzymes, fungi can aid in inhibiting the growth of undesirable microbes, diseases, or competitive algae. This improves the stability of the microalgal culture and lowers the possibility of contamination.
Enhanced biomass production
Fungi have the ability to supply organic materials and growth-enhancing chemicals to microalgae, hence encouraging their growth. In addition, certain fungi can benefit microalgae by eliminating harmful compounds, lessening oxidative stress, and boosting biomass production.
Lipid accumulation
Certain fungi can increase the amount of lipid that accumulates in microalgae, which makes them a more appealing source for the biodiesel generation. Fungal-assisted lipid accumulation has the potential to increase microalgae's total lipid content and increase their feedstock efficiency for biofuel [59].
CO2 utilization
Utilizing and capturing carbon can be facilitated by fungal co-cultivation. Microalgae can use CO2 from fungi's respiration and consumption of organic carbon sources for photosynthesis. This can be very helpful in reducing CO2 emissions [60].
Microalgae-macroalgae co-cultivation
The Micro-Macro Algae In order to promote a mutualistic relationship, co-cultivation entails the simultaneous growing of macro- and microalgae in a shared habitat [61].
In contrast to certain co-cultivation techniques, which favor one organism over the other, this method is typified by the shared benefits that both macro- and microalgae derive from the partnership. Some benefits of Microalgae-Macroalgae Co-cultivation are:
Nutrient complementarity
The nutritional needs of macroalgae and microalgae are different. While macroalgae can use organic resources like dissolved organic matter, microalgae are more adept at absorbing inorganic nutrients like phosphates and nitrates. In the co-cultivation system, their complementary nutrient utilisation reduces competition and improves nutrient cycling [62].
Contaminant control
Because macroalgae compete with infections and undesired bacteria for nutrients, their presence can help inhibit their growth. As a result, there is less chance of contamination and the microalgal culture is more stable.
Enhanced biomass production
Microalgae can adhere to the physical structure provided by macroalgae to form a three-dimensional habitat. This arrangement maximizes light exposure, minimises self-shading, and encourages the growth of microalgae. Greater potential for biofuels results from increased biomass production.
Carbon dioxide utilization
Carbon dioxide (CO2) can be effectively captured and utilized by the co-cultivation method. While macroalgae take up CO2 through respiration, microalgae absorb it during photosynthesis. CO2 emissions can be reduced using two methods of carbon capture.
Microalgae-yeast co-cultivation
In microalgae-yeast co-cultivation, both microalgae and yeast are grown concurrently in a same medium, promoting a mutualistic relationship between the two microbes. The fact that yeast and microalgae gain from this collaboration is its defining feature [63]. Some benefits of Microalgae-Yeast Co-cultivation include:
Nutrient complementarity
Yeast and microalgae require different nutrients. While yeast can eat organic carbon sources like sugars and glycerol, microalgae are more effective at absorbing inorganic nutrients like phosphates and nitrates. Within the co-cultivation system, this complementarity in nutrient utilization reduces competition and improves nutrient cycling [64].
Contaminant control
By competing for available resources, yeast can help inhibit the growth of undesirable microbes or infections. As a result, there is less chance of contamination and the microalgal culture is more stable.
Enhanced biomass production
Growth-promoting compounds, including vitamins and growth factors, can be secreted by yeast, which in turn promotes the growth of microalgae. More biomass is produced as a result, which can be turned into biofuels [65].
Lipid accumulation
Yeast can promote the accumulation of lipids in microalgae by giving them sources of organic carbon to synthesize lipids. Microalgae with higher lipid content as a result are more appealing as feedstock for the generation of biodiesel.
CO2 utilization
During photosynthesis, microalgae may absorb carbon dioxide (CO2), while yeast can make CO2 by using organic carbon sources. This effective usage of CO2 reduces greenhouse gas emissions [66].
Biodiesel production and quality
Biodiesel is a type of diesel fuel made of long-chain fatty acid esters extracted from plants or animals. As a renewable and eco-friendly substitute for conventional diesel derived from petroleum, biodiesel has drawn a lot of interest as a long-term energy source [67].
The efficient utilization and ecological advantages of biodiesel are contingent upon its production and quality. Transesterification is the process by which fats and oils are transformed into glycerin and biodiesel during the manufacturing of biodiesel. The raw materials used, the method of manufacturing, and the chemical characteristics of the biodiesel molecule all affect the quality of biodiesel that is generated [68].The following are important factors to take into account when creating and evaluating biodiesel:
Extraction and transesterification process
The extraction of triglycerides, or fats or oils, from feedstocks and the subsequent transesterification to turn these triglycerides into biodiesel are the two main steps in the manufacturing of biodiesel.
Extraction process
Feedstock Selection: A variety of feedstock, such as discarded cooking oils, animal fats, and vegetable oils (such as soybean, canola, and palm oil), can be utilized to make biodiesel. The resulting biodiesel's characteristics may change depending on the feedstock selection.
Pretreatment: To eliminate contaminants, feedstock may need to be pretreated. Degumming, neutralization, and elimination of free fatty acids are typical pretreatment procedures. The type of feedstock can determine the needed pretreatment.
Methods of Extraction: The feedstock can be extracted from the triglycerides using a variety of techniques, such as:
- Mechanical pressing: Pressuring nuts or seeds to release their oil.
- Solvent extraction: Dissolving the oil in a solvent (such as hexane) and then removing the solvent.
- Supercritical fluid extraction: Oil is extracted using supercritical CO2.
The type of feedstock and other parameters influence the extraction process selection.
The separation of glycerol: The useful byproduct glycerol is present in the extracted oil. It is usually extracted from the oil and has a number of industrial uses [69].
The Transesterification Process
Components: Triglycerides that have been extracted from the feedstock and an alcohol-typically methanol or ethanol- in the presence of a catalyst are the primary components of transesterification. Potassium hydroxide (KOH) and sodium hydroxide (NaOH) are the most widely used catalysts.
Transesterification Reaction: This reaction creates biodiesel (fatty acid methyl or ethyl esters) and glycerol as a byproduct by substituting methanol or ethanol for the glycerol in the triglycerides. One way to depict the chemical reaction is as follows: Biodiesel + Glycerol + 3 Methanol (or Ethanol) -> Triglyceride + 3.
Process Steps: The following steps are usually involved in the transesterification process:
- Mixing: To create a homogeneous solution, the triglycerides, alcohol, and catalyst are combined.
- Reaction: To speed up the transesterification reaction, the mixture is heated and stirred.
- Separation: As a result of their differing densities, the biodiesel and glycerol separate from the mixture after it has been allowed to settle during the reaction.
- Washing: Any leftover contaminants, such as glycerol and leftover catalyst, are eliminated from the crude biodiesel through washing.
Glycerol Separation: The transesterified glycerol is isolated from the biodiesel and can be processed further for use in other processes [70].
Post-Processing: To remove any residual water, deodorize to eliminate aromas, and filter out any particle matter, the crude biodiesel may go through extra processing processes.
Biodiesel quality standards
Biodiesel quality standards are necessary to ensure that biodiesel satisfies particular requirements for effective combustion, lower emissions, and compatibility with current diesel engines [71]. The aforementioned criteria establish the structure for the manufacturing, examination, and application of biodiesel, guaranteeing that it fulfills its potential as an eco-friendly and sustainable fuel.
Ester content
Biodiesel (B100) must have a minimum of 96.5% ester, according to ASTM D6751. Better biodiesel quality is indicated by a higher ester concentration.
Acid value
A value more than 0.50 mg KOH/g is not acceptable. Higher acid values may be a sign of contaminants or free fatty acids, which can cause corrosion and engine deposits.
Moisture content
500 mg/kg (parts per million, ppm) is the highest amount of moisture that can be present. An excessive amount of moisture might cause emulsion development, which is not good for biodiesel [72].
Viscosity
At 40 °C, the viscosity of biodiesel should range from 1.9 to 6.0 mm2/s (cSt). In fuel injection systems, poor atomization can result from excessive viscosity. The sulfur content of biodiesel needs to be below 15 parts per million in orders to comply with environmental regulations. One of the components of toxic emissions is sulfur.
Cold flow qualities
To avoid gelling and filter blockage during cold weather, ASTM D6751 specifies cold flow qualities. The cloud point, for instance, should not be higher than 6 °C (42.8 °F) [73].
Cetane number
To guarantee good ignition quality and enhance engine efficiency, a cetane number of at least 47 is necessary.
Oxidative stability
To avoid deterioration and deposit formation during usage and storage, biodiesel needs to have strong oxidative stability.
Total glycerin content
No more than 0.24% of the total should be glycerin. Overuse of glycerin can cause filter blockage, piston ring sticking, and injector coking.
Density
To prevent modifications in engine fuel supply rates, biodiesel density should be between 0.86 and 0.90 g/cm3 [74].
Significance of biodiesel quality standards:
Engine performance: Engine deposits, corrosion, and wear are minimized and efficient engine performance is ensured by premium biodiesel.
Emission reduction
Particulate matter, nitrogen oxides, and sulfur compounds are just a few of the dangerous pollutants that can be reduced by adhering to quality standards.
Fuel compatibility
Diesel engines and infrastructure that are currently in use may run on biodiesel that satisfies quality standards.
Regulatory compliance
To guarantee the safe and responsible use of biodiesel, regulatory bodies frequently demand adherence to quality requirements.
Environmental benefits
Reducing greenhouse gas emissions and the environmental impact of transportation are two benefits of using high-quality biodiesel [75].
Economic consideration
Microalgae have a number of benefits over conventional crops and the potential to be a substantial source of biomass and biofuel. It is necessary to take into account certain economic factors in order to make the production of biofuel from microalgae financially feasible [76]. The following are the main economic variables and difficulties related to using microalgae as a feedstock for biomass and biofuels:
Investment outlay
The high capital expenditures of establishing the required infrastructure are one of the main economic obstacles to the production of microalgal biofuel. Large-scale microalgal cultivation systems, like photobioreactors or open ponds, need expensive equipment, land, and technological inputs to set up and operate. Smaller enterprises may find it difficult to enter the market due to these upfront costs, which may take years to recover [77].
Operational costs
Growing microalgae requires a lot of energy and the supply of light, nutrients, and carbon dioxide. Maintaining the necessary nutrition supply, ambient conditions, and monitoring systems can come with substantial operational expenditures. Moreover, the ongoing upkeep and observation of microalgal cultures raises the operational expenses.
Costs associated with nutrients
For growth, microalgae require particular nutrients, such as phosphorus and nitrogen. One important economic consideration may be the price of obtaining these nutrients and guaranteeing a steady supply of them. In an attempt to cut expenses, some research is concentrating on creating more environmentally friendly fertilizer sources or recovering nutrients from wastewater [78].
Harvesting and dewatering
Harvesting microalgae and dewatering them pose further financial difficulties. Centrifugation and filtration are two effective but expensive ways to separate microalgae from the growing medium. One of the main areas of research is creating harvesting methods that are both economical and energy-efficient [79].
Lipid extraction
Lipids, or oils, must be extracted from microalgae to produce biofuel. Lipid extraction techniques can be costly, thus it is important from an economic standpoint to optimize the process to maximize lipid recovery while lowering expenses.
Productivity and yield
Achieving high yields and productivity is necessary for the microalgal biomass and biofuel production to be economically viable. Enhancing microalgae's growth rate and lipid content is one of the main objectives of this field of study. To increase yield, improved strains, genetic engineering, and culture techniques are being investigated [80].
Scaling Up
Transforming laboratory or pilot-scale microalgal production into commercial-scale operations presents a challenging economic landscape. To guarantee a smooth transition, the engineering and logistical components of large-scale cultivation should be properly taken into account.
Market competition
The characteristics of the market have an impact on the economics of producing microalgal biofuel. The cost and availability of rival feedstocks for biofuels, like soybean or palm oil, affect how profitable microalgal biofuels can be. Governmental rewards, subsidies, and policies can play a significant role in promoting microalgal biofuel development [81].
Co-product development
Co-products can be produced in addition to biofuels to increase the economic appeal of microalgal biofuel production. High-value biochemicals, animal feeds, and fertilizers are examples of co-products that can assist reduce production costs and make extra money.
Co-cultivation's technological advancements
Enhancing biomass and biofuel production through co-cultivation of microalgae with other microorganisms or macroorganisms has become a viable approach. Co-cultivation systems are now much more viable and efficient because to a number of technical advancements [82]. We examine significant technological advancements in co-cultivation for the production of biomass and biofuels.
Co-cultivation using photobioreactors
Photobioreactors are specialized equipment used to cultivate microorganisms that use photosynthesis, like cyanobacteria and microalgae. Photobioreactors provide a controlled environment for cultivating various microorganisms together in the co-cultivation context. Because these systems can monitor parameters, optimize growth conditions, and increase output, they have become an essential technology in the production of biomass and biofuel [83]. We explore the application of photobioreactors in co-cultivation techniques below.
Photobioreactor types
Photobioreactors with Closed Systems: Photobioreactors with closed systems are hermetically sealed vessels designed to keep microorganisms away from their surroundings. These technologies avoid contamination and are very controlled. Reactors with bubble columns, flat panels, and tubular forms are common.
Open ponds: Used for large-scale cultivation and capable of supporting co-cultivation, open ponds are not enclosed like closed-system photobioreactors. These systems, which are frequently used for both commercial production and research, have less control than closed photobioreactors
Hybrid systems: To maximize growing conditions, hybrid systems incorporate components of both closed and open systems. To provide a controlled environment for initial growth prior to shifting to open systems, closed photobioreactors, for instance, might be connected to open ponds.
Advantages of Co-Cultivation with photobioreactors
Accurate management: Photobioreactors provide accurate management of several environmental parameters, such as light, temperature, pH, and nutrient availability. In co-cultivation, this management is essential for maximizing the growth of several microorganisms with various needs [84].
Contamination prevention
By successfully keeping out undesirable bacteria, closed-system photobioreactors enable the co-cultivation of certain strains without hindrance.
Enhanced output
Photobioreactors can greatly boost the biomass and biofuel output of co-cultured microorganisms by preserving ideal growth conditions.
Year-round culture
Since photobioreactors are not reliant on changes in sunlight and temperature throughout the year, year-round culture is possible. This feature is especially helpful for areas with different seasons.
Monitoring and data gathering
Systems for monitoring and data gathering are built into advanced photobioreactors, giving users access to real-time data on culture parameters. For process optimization and research, this data is invaluable [85].
Closed systems and monitoring
Systems for efficient monitoring and control are essential for maximizing the production of biofuel and microalgal biomass. These technologies give operators access to real-time data, enabling them to maintain optimal growth conditions, make well-informed decisions, and increase production [86], the essential elements, and capabilities of systems for monitoring and controlling.
Sensors for the environment
Environmental sensors are the first line of defense in monitoring, continuously measuring and recording a range of characteristics. These sensors are positioned inside or outside the cultivation system and offer information on variables like:
Light intensity: In closed systems, sensors that measure incoming light can be used to alter artificial light sources for the best possible photosynthesis.
Temperature: Keeping an eye on temperature is essential as it impacts growth rates and may signal possible stress to microalgae [87].
pH Levels: To maximize microalgal development and biofuel generation, the pH must be maintained at the right level.
Nutrient concentrations: By monitoring nutrient levels, sensors make sure microalgae have access to enough resources to thrive.
CO2 Concentration: Since CO2 is a necessary nutrient for photosynthesis, it is vital to keep an eye on its levels.
Data collection and analysis
Real-time data collection and analysis are done using environmental sensor data. The data is processed by sophisticated software tools, which enable operators to identify patterns, anticipate outcomes, and act fast when conditions deviate from ideal ones. Making educated judgments about adding nutrients, regulating pH, or changing light levels is made easier with the use of data analysis [88].
Automation and control
An essential part of systems for monitoring and controlling data is automation. Automation systems can modify a number of factors based on the data and analysis to maintain optimal growth conditions [89].
Tracking algal increase
Various techniques are utilized to gauge the increase of microalgal biomass:
Measurement of optical density: By examining light absorption and scattering, optical sensors can ascertain the density of microalgal biomass. The information here can be useful to track growth trends [90].
Chlorophyll fluorescence: By measuring chlorophyll fluorescence, fluorometers can reveal information about stress levels and photosynthetic activity as shown in Fig 2 .
Cell counting and morphology analysis: Microalgae cells can be imaged using sophisticated imaging technologies, enabling the counting and morphological analysis of the cells.
Contaminant detection: Unwanted microbes and pollutants are also detected by monitoring systems. Accordingly, resource rivalry is avoided and the purity of the microalgal culture is preserved. When pollutants are found, automated detection systems have the ability to initiate interventions.
Feedback control loops
These systems allow for automated modifications in response to gathered data. By maintaining cultivation conditions within ideal ranges, these loops maximize the growth of microalgae and the production of biofuel [91].
Remote monitoring and control
A lot of systems have the ability to monitor and control remotely, giving users the ability to view and modify cultivation parameters from a distance. It is valuable for large scale and outdoor cultivation systems.
Data storage and historical analysis
In addition to store historical data, monitoring and control systems allow for the long-term examination of performance, trends, and growth patterns. Future cultivation strategy decisions can be made with the help of this data [92].
Co-cultivation using genetic engineering
Enhancing microalgae's potential for biomass and biofuel production is mostly dependent on genetic engineering. Co-cultivating microalgae with other microorganisms- especially modified strains- can greatly increase biomass quality, output of biofuel, and productivity [93]. Genetic engineering applications in co-cultivation methods are useful for producing microalgal biomass and biofuel.
Enhanced production of lipids: Modifying microalgal strains through genetic engineering makes it possible to increase lipid (oil) synthesis, which is necessary for the biofuel manufacture. Among the strategies are: To boost the synthesis of triglycerides, the main lipid source for biodiesel, researchers can overexpress important genes involved in lipid biosynthesis. To direct more carbon into the synthesis of lipids, genetic alteration can optimize the pathways involved in lipid biosynthesis [94]. By engineering microalgae to accumulate lipids in response to stressors like phosphate or nitrogen deficiency, lipid content can be increased.
Stress tolerance: In culture, microalgae are frequently subjected to environmental stresses that might impede their growth and output. Stress tolerance can be increased by genetic engineering to increase the resilience of microalgae, genes related to stress responses, such as resistance to salinity or drought can be added.
CO2 fixation: Microalgae that have been genetically modified can be enhanced for effective CO2 fixation, which is necessary for the generation of biomass and biofuel. This entails lowering CO2 loss and raising rates of carbon uptake [95].
Nitrogen fixation: In co-cultivation systems, tailored strains may fix ambient nitrogen, minimizing the need for nitrogen supplementation. Microalgae need nitrogen to develop.
Co-cultivation symbiosis: Microalgae and co-cultured microorganisms can form symbiotic interactions through genetic engineering. For example, microalgae can be genetically modified to supply particular nutrients or chemicals to their co-cultured partners in return for advantageous goods or services [96].
Growth enhancement: To ensure quick biomass production, genetic engineering can be employed to increase microalgal growth rates. This involves maximizing photosynthetic efficiency and cell division.
Modified metabolism: It is possible to create engineered strains that modify the microalgae's metabolic pathways to emphasize the synthesis of particular molecules, including high-value biochemicals or biofuels.
Resistance to pollutants: By introducing resistance to pathogens or pollutants through genetic engineering, the microalgal culture can be shielded from undesirable bacteria and resource competition is avoided [97].
Improved co-cultivation compatibility: Microalgae and co-cultured microorganisms can coexist more harmoniously thanks to genetic engineering, promoting the growth and generation of biomass and biofuels from both partners.
Controllable genetic switches: They are a feature of engineered microalgae that enable the exact regulation of particular metabolic activities or pathways in response to the environmental circumstances or cultivation needs.
Regulatory considerations: When it comes to genetically modified microalgae, safety and regulatory issues must be taken into account. Obtaining permission and adhering to applicable laws are essential, particularly when introducing genetically engineered microbes into public spaces [98].
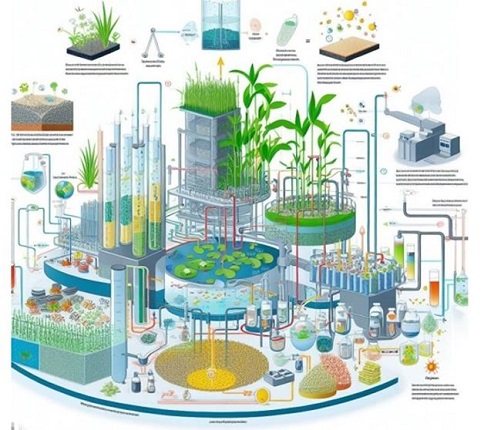
Impact of Co-cultivation on microalgal growth
Improved biomass production
Co-cultivation with certain microorganisms can lead to improved microalgal biomass production. This enhancement is often attributed to the provision of growth-promoting factors, such as vitamins, growth hormones, or organic matter, by the co-cultivated organisms [99]. In addition, some microorganisms can help protect microalgae from environmental stressors, which can further contribute to increased biomass production. Co-cultivation has shown significant potential for improving microalgal biomass production through several mechanisms:
Mutualism and synergy: Co-cultivation can create mutualistic interactions between microalgal species, leading to improved biomass production. For instance, co-cultures of nitrogen-fixing cyanobacteria and non-nitrogen-fixing microalgae can benefit from nitrogen transfer, resulting in enhanced growth [100]. Such mutualistic relationships can also reduce competition for resources and create a more stable growth environment.
Resource partitioning: Co-cultures allow for the utilization of complementary resources, reducing competition and maximizing resource efficiency. Microalgae with distinct nutrient preferences can be combined to ensure efficient nutrient utilization [101]. This resource partitioning contributes to higher overall biomass production.
Biofloc formation: Co-cultivation can promote the formation of microalgal bioflocs, which enhance biomass production. Bioflocs are composed of microalgae and bacteria, creating a microenvironment that can facilitate nutrient cycling, protect microalgae from predation, and reduce shear stress [102].
Enhanced lipid content and quality
Co-cultivation has been shown to influence lipid production in microalgae positively:
Metabolic interactions: Co-cultivation can lead to metabolic interactions between microalgal species, resulting in increased lipid content. For example, co-culturing Chlorella and Scenedesmus has been found to enhance the production of lipids in both species, attributed to cross-species metabolite exchange [103].
Induction of stress responses: Co-cultivation can induce stress responses in microalgae, leading to higher lipid accumulation. Competition for resources and allelopathic interactions between co-cultured species can stimulate lipid production as a defense mechanism [104].
Nutrient utilization and recycling
Co-cultivation can improve nutrient utilization efficiency and recycling:
Nutrients recycling: In co-cultivation systems, nutrients excreted by one microalgal species can be used by others, reducing the need for external nutrient supplementation [105]. This recycling minimizes the environmental impact and the cost associated with nutrient provision.
Nitrogen and phosphorus removal: Co-cultivation can be applied for wastewater treatment. Co-cultures can efficiently remove nitrogen and phosphorus from wastewater, making it a sustainable solution for nutrient removal and water purification [106].
Environmental and nutrient considerations
Some environmental and nutrient considerations associated with microalgal co-cultivation are as follow:
Nutrient exchange and competition in co-cultivation: In a co-cultivation system, there can be nutrient exchange and competition between microalgae and co-cultivated organisms. For instance, microalgae may compete with bacteria or other microorganisms for essential nutrients such as nitrogen and phosphorus [107]. The competition for nutrients may influence the growth dynamics and overall productivity of the co-culture.
Carbon dioxide utilization in co-cultivation: Co-cultivation systems can contribute to carbon dioxide (CO2) mitigation and utilization as shown in Fig 3. Microalgae, when co-cultivated with other organisms, can efficiently capture and fix CO2 from various sources, such as industrial emissions or flue gases. The ability of microalgae to sequester CO2 can have implications for reducing greenhouse gas emissions [108].
Wastewater treatment applications: Co-cultivation of microalgae with bacteria or other microorganisms has shown promise in wastewater treatment applications. The microorganisms in the co-culture can help in the removal of organic pollutants, nitrogen, and phosphorus from wastewater, providing a cost-effective and sustainable approach for wastewater treatment [109].
Sustainability and future prospects
Sustainability of Co-cultivation systems
Resource efficiency: Co-cultivation systems offer the potential for improved resource efficiency. They can enhance nutrient utilization and reduce the need for external nutrient supplementation in microalgal growth. This is particularly important in the context of sustainable agriculture and aquaculture, where microalgae can be used to supplement the nutrient requirements of crops and aquatic organisms, reducing the reliance on synthetic fertilizers [110].
Carbon sequestration: Co-cultivation systems, especially those involving microalgae, have the capacity to sequester carbon dioxide (CO2) from industrial emissions and other sources. This contributes to mitigating greenhouse gas emissions and can support carbon capture and utilization (CCU) efforts, aligning with global sustainability goals [111].
Waste utilization: Co-cultivation can provide a sustainable solution for utilizing organic waste streams, such as agricultural and industrial effluents. By co-cultivating microalgae with waste-treating microorganisms, organic matter can be efficiently converted into valuable biomass and bioenergy, reducing waste disposal and pollution [112].
Potential for commercial-scale production
Biofuel production: Co-cultivation systems are increasingly explored for commercial-scale biofuel production. The enhanced lipid content and growth rates achieved through co-cultivation can make microalgae a competitive feedstock for biodiesel and biojet fuel production, with the potential to reduce reliance on fossil fuels [113].
High-value products: Beyond biofuels, co-cultivation can be harnessed for the production of high-value compounds, such as pharmaceuticals, nutraceuticals, and bioplastics. The versatility of co-cultivation systems allows for the tailored production of specific compounds, offering opportunities for the pharmaceutical and biotechnology industries [114-117].
Sustainable agriculture: Co-cultivation has applications in sustainable agriculture by providing biofertilizers, biostimulants, and disease control agents. The co-cultivation of microalgae with beneficial microorganisms can enhance soil health, nutrient availability, and crop yield, supporting eco-friendly farming practices.
Wastewater treatment: Large-scale co-cultivation systems for wastewater treatment can serve municipalities and industries, treating wastewater while producing valuable biomass. The dual-purpose nature of these systems can make them economically viable and environmentally sustainable [118].
Conclusion
The exploration of emerging co-cultivation strategies for microalgal biomass and biodiesel production has unfolded a multifaceted landscape that holds promise for the sustainable future of biofuel technologies. This study embarked on a comprehensive journey, delving into various dimensions of co-cultivation approaches, biodiesel production processes, and the overarching impact on microalgal growth.
The foundational sections delved into the intrinsic characteristics of microalgae as a source of biodiesel, emphasizing the significance of their lipid production capabilities.
The subsequent exploration of co-cultivation approaches, ranging from bacterial and fungal co-cultivation to innovative combinations with macroalgae and yeast, demonstrated a nuanced understanding of the diverse synergies that can be harnessed for enhanced microalgal productivity. As the study unfolded the intricacies of biodiesel production and quality standards, it became evident that co-cultivation offers not only technological advancements, but also economic considerations crucial for the scalability of biofuel production.
The examination of technological advancements of co-cultivation, including the use of photobioreactors, closed systems with monitoring capabilities, and genetic engineering, showcased a commitment to cutting-edge methodologies. The impact of co-cultivation on microalgal growth emerged as a focal point, with tangible improvements observed in biomass production, lipid content, and nutrient utilization.
The study underscored the potential of co-cultivation to not only enhance biodiesel-relevant traits, but also contribute to environmental sustainability through nutrient recycling. Concerning the broader environmental and nutrient considerations, the study addressed the intricate balance required for successful co-cultivation systems, paving the way for a more holistic approach to biofuel production. The sustainability and future prospects section illuminated the potential of co-cultivation systems to transition from experimental setups to commercially viable production, bringing biofuels closer to mainstream adoption.
This study contributes significantly to the evolving discourse on microalgal biomass and biodiesel production by championing the cause of co-cultivation strategies. As we navigate towards a future where sustainable energy solutions are imperative, the findings of this research provide valuable insights, laying the groundwork for a more efficient, economically viable, and environmentally conscious approach to microalgal biofuel production. The journey from microalgal characteristics to co-cultivation strategies and their far-reaching implications concludes with sustainability vision.
ORCID
Merit Oluchi Ori
https://orcid.org/0009-0005-9259-5842
Francis-Dominic Makong Ekpan
https://orcid.org/0009-0009-4822-7005
Humphrey Sam Samuel
https://orcid.org/0009-0001-7480-4234
Odii Peter Egwuatu
https://orcid.org/0009-0004-0662-9077
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Declarations
Conflict of interest: The authors have no relevant financial or non-financial interests to disclose.
Ethical approval: Not applicable.
Consent to participate: Not applicable.
Consent for publication: Not applicable
--------------------------------------------------------------------------------------------------------------------------------------------------
OPEN ACCESS
©2024 The author(s). This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit: http://creativecommons.org/licenses/by/4.0/
PUBLISHER NOTE
Sami Publishing Company remains neutral concerning jurisdictional claims in published maps and institutional affiliations.
CURRENT PUBLISHER
Sami Publishing Company
- Lardon L, Hélias A, Sialve B, Steyer JP, Bernard O. Life-cycle assessment of biodiesel production from microalgae.Environmental Science & Technology, 2009; 43(17):6475-6481. [Crossref], [Google Scholar], [Publisher]
- Crutzen PJ, Mosier AR, Smith KA, Winiwarter W. N 2 O release from agro-biofuel production negates global warming reduction by replacing fossil fuels. Paul J. Crutzen: A pioneer on atmospheric chemistry and climate change in the anthropocene. 2016:227-38. [Crossref], [Google Scholar], [Publisher]
- Huntley ME, Redalje DG. CO 2 mitigation and renewable oil from photosynthetic microbes: a new appraisal. Mitigation and adaptation strategies for global change. 2007 May;12:573-608. [Crossref], [Google Scholar], [Publisher]
- Li Y, Horsman M, Wu N, Lan CQ, Dubois‐Calero N. Biofuels from microalgae. Biotechnology progress. 2008 Jul;24(4):815-20. [Crossref], [Google Scholar], [Publisher]
- Kadam KL. Microalgae production from power plant flue gas: environmental implications on a life cycle basis. National Renewable Energy Lab.(NREL), Golden, CO (United States); 2001 Jun 22. [Crossref], [Google Scholar], [Publisher]
- Frischknecht R, Jungbluth N, Althaus HJ, Hischier R, Doka G, Dones R, Heck T, Hellweg S, Wernet G, Nemecek T, Rebitzer G. Overview and methodology. Data v2. 0 (2007). Ecoinvent report No. 1. Ecoinvent Centre; 2007. [Google Scholar], [Publisher]
- Illman AM, Scragg AH, Shales SW. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme and microbial technology. 2000 Nov 1;27(8):631-5. [Crossref], [Google Scholar], [Publisher]
- Chisti Y. Biodiesel from microalgae beats bioethanol. Trends in biotechnology. 2008 Mar 1;26(3):126-31. [Crossref], [Google Scholar], [Publisher]
- Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: a review. Renewable and sustainable energy reviews. 2010 Jan 1;14(1):217-32. [Crossref], [Google Scholar], [Publisher]
- Aresta M, Dibenedetto A, Barberio G. Utilization of macro-algae for enhanced CO2 fixation and biofuels production: Development of a computing software for an LCA study. Fuel processing technology. 2005 Oct 1;86(14-15):1679-93. [Crossref], [Google Scholar], [Publisher]
- Surendhiran D, Vijay M. Microalgal Biodiesel-A Comprehensive Review on the Potential and Alternative Biofuel. Research Journal of Chemical Sciences, ISSN. 2012;2231:606X. [Google Scholar], [PDF]
- Chisti Y. Biodiesel from microalgae. Biotechnology advances. 2007;25(3):294-306. [Crossref], [Google Scholar], [Publisher]
- Li Y, Horsman M, Wu N, Lan CQ, Dubois‐Calero N. Biofuels from microalgae. Biotechnology progress. 2008;24(4):815-20. [Crossref], [Google Scholar], [Publisher]
- Munoz R, Guieysse B. Algal–bacterial processes for the treatment of hazardous contaminants: a review. Water research. 2006; 40(15):2799-815. [Crossref], [Google Scholar], [Publisher]
- Rengel A, editor Promising technologies for biodiesel production from algae growth systems. 8th European IFSA symposium, Clermont-Ferrand, France; 2008. [Google Scholar], [PDF]
- Kozlovska J, Valančius K, Petraitis E. Sapropel use as a biofuel feasibility studies. Research Journal of Chemical Sciences. 2012; 2(5):29-34. [Google Scholar], [PDF]
- Spolaore P, Joannis-Cassan C, Duran E, Isambert A. Commercial applications of microalgae. Journal of bioscience and bioengineering. 2006; 101(2):87-96. [Crossref], [Google Scholar], [Publisher]
- Richmond A. Handbook of microalgal culture: biotechnology and applied phycology: John Wiley & Sons; 2008. [Crossref], [Google Scholar], [Publisher]
- Etim, E.E., Ugo Nweke-Maraizu., Samuel, H.S. A Review of Theoretical Techniques in Corrosion Inhibition Studies. Communication in Physical Sciences, 2023; 9(4): 394-403. [Google Scholar], [Publisher]
- Buck BH, Buchholz CM. The offshore-ring: a new system design for the open ocean aquaculture of macroalgae. Journal of Applied Phycology. 2004; 16(5):355-68. [Crossref], [Google Scholar], [Publisher]
- Poulíčková A, Hašler P, Lysáková M, Spears B. The ecology of freshwater epipelic algae: an update. Phycologia. 2008 Sep 1;47(5):437-50. [Google Scholar], [Publisher]
- Grobbelaar JU, Bornman CH. Algal biotechnology: real opportunities for Africa. South African Journal of Botany. 2004 Mar 1;70(1):140-4. [Crossref], [Google Scholar], [Publisher]
- Azarpour A, Zendehboudi S, Mohammadzadeh O, Rajabzadeh AR, Chatzis I. A review on microalgal biomass and biodiesel production through Co-cultivation strategy. Energy Conversion and Management. 2022 Sep 1;267:115757. [Crossref], [Google Scholar], [Publisher]
- Zhang S, Zhang L, Xu G, Li F, Li X. A review on biodiesel production from microalgae: Influencing parameters and recent advanced technologies. Frontiers in Microbiology. 2022 Jul 29;13:970028. [Crossref], [Google Scholar], [Publisher]
- Kumar L, Anand R, Shah MP, Bharadvaja N. Microalgae biodiesel: a sustainable source of energy, unit operations, technological challenges, and solutions. Journal of Hazardous Materials Advances. 2022 Nov 1;8:100145. [Crossref], [Google Scholar], [Publisher]
- Kumar L, Anand R, Shah MP, Bharadvaja N. Microalgae biodiesel: a sustainable source of energy, unit operations, technological challenges, and solutions. Journal of Hazardous Materials Advances. 2022 Nov 1;8:100145. [Crossref], [Google Scholar], [Publisher]
- Arenas EG, Rodriguez Palacio MC, Juantorena AU, Fernando SE, Sebastian PJ. Microalgae as a potential source for biodiesel production: techniques, methods, and other challenges. International Journal of Energy Research. 2017 May;41(6):761-89. [Crossref], [Google Scholar], [Publisher]
- Tiwari A, Kiran T. Biofuels from microalgae. Advances in biofuels and bioenergy. 2018 Jul 4:239-49. [Google Scholar], [Publisher]
- Sarker PK, Kapuscinski AR, McKuin B, Fitzgerald DS, Nash HM, Greenwood C. Microalgae-blend tilapia feed eliminates fishmeal and fish oil, improves growth, and is cost viable. Scientific reports. 2020 Nov 12;10(1):19328. [Crossref], [Google Scholar], [Publisher]
- Sarker PK, Kapuscinski AR, Vandenberg GW, Proulx E, Sitek AJ. Towards sustainable and ocean-friendly aquafeeds: Evaluating a fish-free feed for rainbow trout (Oncorhynchus mykiss) using three marine microalgae species. Elem Sci Anth. 2020;8:5. [Crossref], [Google Scholar], [Publisher]
- Norambuena F, Hermon K, Skrzypczyk V, Emery JA, Sharon Y, Beard A, Turchini GM. Algae in fish feed: performances and fatty acid metabolism in juvenile Atlantic salmon. PloS one. 2015 Apr 15;10(4):e0124042. [Crossref], [Google Scholar], [Publisher]
- Nagappan S, Das P, AbdulQuadir M, Thaher M, Khan S, Mahata C, Al-Jabri H, Vatland AK, Kumar G. Potential of microalgae as a sustainable feed ingredient for aquaculture. Journal of Biotechnology. 2021 Nov 20;341:1-20. [Crossref], [Google Scholar], [Publisher]
- Kusmayadi A, Leong YK, Yen HW, Huang CY, Chang JS. Microalgae as sustainable food and feed sources for animals and humans–biotechnological and environmental aspects. Chemosphere. 2021 May 1;271:129800. [Crossref], [Google Scholar], [Publisher]
- Li H, Chen S, Liao K, Lu Q, Zhou W. Microalgae biotechnology as a promising pathway to ecofriendly aquaculture: a state‐of‐the‐art review. Journal of Chemical Technology & Biotechnology. 2021 Apr;96(4):837-52. [Crossref], [Google Scholar], [Publisher]
- Lu Q, Li H, Zou Y, Liu H, Yang L. Astaxanthin as a microalgal metabolite for aquaculture: A review on the synthetic mechanisms, production techniques, and practical application. Algal Research. 2021 Apr 1;54:102178. [Crossref], [Google Scholar], [Publisher]
- Beal CM, Gerber LN, Sills DL, Huntley ME, Machesky SC, Walsh MJ, Tester JW, Archibald I, Granados J, Greene CH. Algal biofuel production for fuels and feed in a 100-ha facility: A comprehensive techno-economic analysis and life cycle assessment. Algal Research. 2015 Jul 1;10:266-79. [Crossref], [Google Scholar], [Publisher]
- Camacho-Rodríguez J, Macías-Sánchez MD, Cerón-García MC, Alarcón FJ, Molina-Grima E. Microalgae as a potential ingredient for partial fish meal replacement in aquafeeds: nutrient stability under different storage conditions. Journal of Applied Phycology. 2018 Apr;30:1049-59. [Crossref], [Google Scholar], [Publisher]
- Ahmad A, Banat F, Alsafar H, Hasan SW. Recent breakthroughs in integrated biomolecular and biotechnological approaches for enhanced lipid and carotenoid production from microalgae. Phytochemistry Reviews. 2023 Aug;22(4):993-1013. [Crossref], [Google Scholar], [Publisher]
- Ori O, Edet I, Ekpan M, Samuel S, Egwuatu P, Ajor J. Revisiting on Applications of Industrial Filters in Enhancing Polymer Product Quality and Performance. Eurasian Journal of Science and Technology, 2024; 4(2): 116-132. [Crossref], [Google Scholar], [Publisher]
- Pavithra KG, Kumar PS, Jaikumar V, Vardhan KH, SundarRajan P. Microalgae for biofuel production and removal of heavy metals: a review. Environmental Chemistry Letters. 2020 Nov;18:1905-23. [Crossref], [Google Scholar], [Publisher]
- Nidhina N, Muthukumar SP. Antinutritional factors and functionality of protein-rich fractions of industrial guar meal as affected by heat processing. Food Chemistry. 2015 Apr 15;173:920-6. [Crossref], [Google Scholar], [Publisher]
- Ullah Z, Ahmed G, un Nisa M, Sarwar M. Standardized ileal amino acid digestibility of commonly used feed ingredients in growing broilers. Asian-Australasian journal of animal sciences. 2016 Sep;29(9):1322. [Crossref], [Google Scholar], [Publisher]
- García-Vaquero M, Hayes M. Red and green macroalgae for fish and animal feed and human functional food development. Food Reviews International. 2016 Jan 2;32(1):15-45. [Crossref], [Google Scholar], [Publisher]
- Samuel HS, Ekpan FM. Revolutionizing Drugs Administration: Techniques in Drug Delivery and Development. Internationak Journal of Biochemistry Physiology. 2023; 8(2):000237. [Crossref], [Publisher]
- Marques A, Dhont J, Sorgeloos P, Bossier P. Evaluation of different yeast cell wall mutants and microalgae strains as feed for gnotobiotically grown brine shrimp Artemia franciscana. Journal of Experimental Marine Biology and Ecology. 2004 Nov 25;312(1):115-36. [Crossref], [Google Scholar], [Publisher]
- Bosch G, Zhang S, Oonincx DG, Hendriks WH. Protein quality of insects as potential ingredients for dog and cat foods. Journal of nutritional science. 2014;3:e29. [Crossref], [Google Scholar], [Publisher]
- Aladetohun NF, Sogbesan OA. Utilization of blood meal as a protein ingredient from animal waste product in the diet of Oreochromis niloticus. International journal of fisheries and aquaculture. 2013 Sep;5(9):234-7. [Crossref], [Google Scholar], [PDF]
- Hussain SM, Afzal M, Salim M, Javid A, Khichi TA, Hussain M, Raza SA. Apparent digestibility of fish meal, blood meal and meat meal for Labeo rohita fingerlings. Journal of Animal and Plant Sciences. 2011 Jan 1;21(4):807-11. [Google Scholar], [PDF]
- Ahmad A, W. Hassan S, Banat F. An overview of microalgae biomass as a sustainable aquaculture feed ingredient: Food security and circular economy. Bioengineered. 2022 Apr 1;13(4):9521-47. [Crossref], [Google Scholar], [Publisher]
- Sundaram T, Rajendran S, Gnanasekaran L, Rachmadona N, Jiang JJ, Khoo KS, Show PL. Bioengineering strategies of microalgae biomass for biofuel production: recent advancement and insight. Bioengineered. 2023 Dec 31;14(1):2252228. [Crossref], [Google Scholar], [Publisher]
- Samuel S, Etim E, Shinggu P, Bako B. Machine Learning in Thermochemistry: Unleashing Predictive Modelling for Enhanced Understanding of Chemical Systems. Communication in Physical Sciences, 2024 April 06; 11(1), 47-75 [Crossref], [Google Scholar], [Publisher]
- Ray A, Nayak M, Ghosh A. A review on co-culturing of microalgae: A greener strategy towards sustainable biofuels production. Science of the Total Environment. 2022 Jan 1;802:149765. [Crossref], [Google Scholar], [Publisher]
- Zhu L. Microalgal culture strategies for biofuel production: a review. Biofuels, Bioproducts and Biorefining. 2015 Nov;9(6):801-14. [Crossref], [Google Scholar], [Publisher]
- Han J, Zhang L, Wang S, Yang G, Zhao L, Pan K. Co-culturing bacteria and microalgae in organic carbon containing medium. Journal of Biological Research-Thessaloniki. 2016 Dec;23(1):1-9. [Crossref], [Google Scholar], [Publisher]
- Edet PI, Samuel HS. A review of antioxidant applications and phytochemical constituents of Anacardium Occidentale leaf extract. Faculty of Natural and Applied Sciences Journal of Scientific Innovations. 2023 Dec 30;5(1):15-20. [Google Scholar], [Publisher]
- Yao S, Lyu S, An Y, Lu J, Gjermansen C, Schramm A. Microalgae–bacteria symbiosis in microalgal growth and biofuel production: a review. Journal of applied microbiology. 2019 Feb 1;126(2):359-68. [Crossref], [Google Scholar], [Publisher]
- Onyeaka H, Ekwebelem OC. A review of recent advances in engineering bacteria for enhanced CO2 capture and utilization. International Journal of Environmental Science and Technology. 2023 Apr;20(4):4635-48. [Crossref], [Google Scholar], [Publisher]
- Santo ÉD, Ishii M, Pinto UM, Matsudo MC, Carvalho JC. Obtaining bioproducts from the studies of signals and interactions between microalgae and bacteria. Microorganisms. 2022 Oct 14;10(10):2029. [Crossref], [Google Scholar], [Publisher]
- Sharma KK, Schuhmann H, Schenk PM. High lipid induction in microalgae for biodiesel production. Energies. 2012 May 18;5(5):1532-53. [Crossref], [Google Scholar], [Publisher]
- Wrede D, Taha M, Miranda AF, Kadali K, Stevenson T, Ball AS, Mouradov A. Co-cultivation of fungal and microalgal cells as an efficient system for harvesting microalgal cells, lipid production and wastewater treatment. PloS one. 2014 Nov 24;9(11):e113497. [Crossref], [Google Scholar], [Publisher]
- González-González LM, de-Bashan LE. Toward the enhancement of microalgal metabolite production through microalgae–bacteria consortia. Biology. 2021 Apr 1;10(4):282. [Crossref], [Google Scholar], [Publisher]
- Li Y, Li L, Yu Y, Hu Q, Li X. Impact of dietary protein content on soil bacterial and fungal communities in a rice–crab co-culture system. Frontiers in Microbiology. 2021 Jun 21;12:696427. [Crossref], [Google Scholar], [Publisher]
- Kapoore RV, Padmaperuma G, Maneein S, Vaidyanathan S. Co-culturing microbial consortia: approaches for applications in biomanufacturing and bioprocessing. Critical Reviews in Biotechnology. 2022 Jan 2;42(1):46-72. [Crossref], [Google Scholar], [Publisher]
- Postma JA, Lynch JP. Complementarity in root architecture for nutrient uptake in ancient maize/bean and maize/bean/squash polycultures. Annals of botany. 2012 Jul 1;110(2):521-34. [Crossref], [Google Scholar], [Publisher]
- Szotkowski M, Holub J, Šimanský S, Hubačová K, Sikorová P, Mariničová V, Němcová A, Márová I. Bioreactor co-cultivation of high lipid and carotenoid producing yeast Rhodotorula kratochvilovae and several microalgae under stress. Microorganisms. 2021 May 28;9(6):1160. [Crossref], [Google Scholar], [Publisher]
- Miranda AM, Hernandez-Tenorio F, Ocampo D, Vargas GJ, Sáez AA. Trends on CO2 capture with microalgae: A bibliometric analysis. Molecules. 2022 Jul 22;27(15):4669. [Crossref], [Google Scholar], [Publisher]
- Kavitha, S., Ravi, Y. K., Kumar, G., & Nandabalan, Y. K. (2024). Microalgal biorefineries: Advancement in machine learning tools for sustainable biofuel production and value-added products recovery. Journal of Environmental Management, 353, 120135.
- Salaheldeen M, Mariod AA, Aroua MK, Rahman SA, Soudagar ME, Fattah IR. Current state and perspectives on transesterification of triglycerides for biodiesel production. Catalysts. 2021 Sep 18;11(9):1121. [Crossref], [Google Scholar], [Publisher]
- Mendow G, Veizaga NS, Sánchez BS, Querini CA. Biodiesel production by two-stage transesterification with ethanol. Bioresource Technology. 2011 Nov 1;102(22):10407-13. [Crossref], [Google Scholar], [Publisher]
- Mumtaz MW, Adnan A, Mukhtar H, Rashid U, Danish M. Biodiesel production through chemical and biochemical transesterification: Trends, technicalities, and future perspectives. InClean energy for sustainable development 2017 Jan 1 (pp. 465-485). Academic Press. [Crossref], [Google Scholar], [Publisher]
- Ekpan, F.M., Ori, M.O, Samuel, H.S., & Egwuatu, O.P. (2024). Emerging Technologies for Eco-Friendly Production of Bioethanol from Lignocellulosic Waste Materials. Eurasian Journal of Science and Technology, (), 179-194. doi: 10.48309/ejst.2024.429106.1119
- Samuel HS, Nweke-Maraizu U, Etim EE. Machine learning for characterizing halogen bonding interactions. Faculty of Natural and Applied Sciences Journal of Scientific Innovations. 2023 Dec 30;5(1):102-14. [Google Scholar], [Publisher]
- Sundaram T, Rajendran S, Gnanasekaran L, Rachmadona N, Jiang JJ, Khoo KS, Show PL. Bioengineering strategies of microalgae biomass for biofuel production: recent advancement and insight. Bioengineered. 2023 Dec 31;14(1):2252228. [Crossref], [Google Scholar], [Publisher]
- Rajvanshi M, Prasad V, Govindachary S, Dasgupta S. Challenges in microalgae cultivation, harvesting and biooil production: prospective in biofuel development for techno economic feasibility under biorefinery stratagem. Bioresource Technology. 2021 Oct 18:126155-.[Crossref], [Google Scholar], [Publisher]
- Moshood TD, Nawanir G, Mahmud F. Microalgae biofuels production: A systematic review on socioeconomic prospects of microalgae biofuels and policy implications. Environmental Challenges. 2021 Dec 1;5:100207. [Crossref], [Google Scholar], [Publisher]
- Sundaram T, Rajendran S, Gnanasekaran L, Rachmadona N, Jiang JJ, Khoo KS, Show PL. Bioengineering strategies of microalgae biomass for biofuel production: recent advancement and insight. Bioengineered. 2023 Dec 31;14(1):2252228. [Crossref], [Google Scholar], [Publisher]
- Satpati GG, Dikshit PK, Mal N, Pal R, Sherpa KC, Rajak RC, Rather SU, Raghunathan S, Davoodbasha M. A state of the art review on the co-cultivation of microalgae-fungi in wastewater for biofuel production. Science of The Total Environment. 2023 Apr 20;870:161828. [Crossref], [Google Scholar], [Publisher]
- Yin Z, Zhu L, Li S, Hu T, Chu R, Mo F, Hu D, Liu C, Li B. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: Environmental pollution control and future directions. Bioresource Technology. 2020 Apr 1;301:122804. [Crossref], [Google Scholar], [Publisher]
- Alazaiza MY, Albahnasawi A, Al Maskari T, Abujazar MS, Bashir MJ, Nassani DE, Abu Amr SS. Biofuel production using cultivated algae: Technologies, economics, and its environmental impacts. Energies. 2023 Jan 26;16(3):1316. [Crossref], [Google Scholar], [Publisher]
- Koller M, Salerno A, Tuffner P, Koinigg M, Böchzelt H, Schober S, Pieber S, Schnitzer H, Mittelbach M, Braunegg G. Characteristics and potential of micro algal cultivation strategies: a review. Journal of Cleaner Production. 2012 Dec 1;37:377-88. [Crossref], [Google Scholar], [Publisher]
- Thoré ES, Schoeters F, Spit J, Van Miert S. Real-time monitoring of microalgal biomass in pilot-scale photobioreactors using nephelometry. Processes. 2021 Aug 28;9(9):1530. [Crossref], [Google Scholar], [Publisher]
- Havlik I, Reardon KF, Ünal M, Lindner P, Prediger A, Babitzky A, Beutel S, Scheper T. Monitoring of microalgal cultivations with on-line, flow-through microscopy. Algal Research. 2013 Jul 1;2(3):253-7. [Crossref], [Google Scholar], [Publisher]
- Höpfner T, Bluma A, Rudolph G, Lindner P, Scheper T. A review of non-invasive optical-based image analysis systems for continuous bioprocess monitoring. Bioprocess and biosystems engineering. 2010 Feb;33:247-56. [Crossref], [Google Scholar], [Publisher]
- Bosma R, van Zessen E, Reith JH, Tramper J, Wijffels RH. Prediction of volumetric productivity of an outdoor photobioreactor. Biotechnology and Bioengineering. 2007 Aug 1;97(5):1108-20. [Crossref], [Google Scholar], [Publisher]
- Nguyen BT, Rittmann BE. Low-cost optical sensor to automatically monitor and control biomass concentration in microalgal cultivation. Algal research. 2018 Jun 1;32:101-6. [Crossref], [Google Scholar], [Publisher]
- Wei, C., Xu, Y., Li, Y., Wei, W., Feng, Y., Li, Z., & Xu, L. (2024). Life-cycle assessment of microalgae liquid biofuel production in biofilm cultivation system via conversion technologies of transesterification, hydrothermal liquefaction and pyrolysis. Journal of Cleaner Production, 436, 140559.
- Barclay W, Apt K. Strategies for bioprospecting microalgae for potential commercial applications. Handbook of microalgal culture: Applied phycology and biotechnology. 2013 May 7:69-79. [Crossref], [Google Scholar], [Publisher]
- Grama SB, Liu Z, Li J. Emerging trends in genetic engineering of microalgae for commercial applications. Marine Drugs. 2022 Apr 24;20(5):285. [Crossref], [Google Scholar], [Publisher]
- Yang B, Liu J, Ma X, Guo B, Liu B, Wu T, Jiang Y, Chen F. Genetic engineering of the Calvin cycle toward enhanced photosynthetic CO2 fixation in microalgae. Biotechnology for biofuels. 2017 Dec;10(1):1-3. [Crossref], [Google Scholar], [Publisher]
- Bajhaiya AK, Dean AP, Driver T, Trivedi DK, Rattray NJ, Allwood JW, Goodacre R, Pittman JK. High-throughput metabolic screening of microalgae genetic variation in response to nutrient limitation. Metabolomics. 2016 Jan;12:1-4. [Crossref], [Google Scholar], [Publisher]
- Doron L, Segal NA, Shapira M. Transgene expression in microalgae—from tools to applications. Frontiers in plant science. 2016 Apr 22;7:505. [Crossref], [Google Scholar], [Publisher]
- Ng IS, Keskin BB, Tan SI. A critical review of genome editing and synthetic biology applications in metabolic engineering of microalgae and cyanobacteria. Biotechnology Journal. 2020 Aug;15(8):1900228. [Crossref], [Google Scholar], [Publisher]
- Kumar G, Shekh A, Jakhu S, Sharma Y, Kapoor R, Sharma TR. Bioengineering of microalgae: recent advances, perspectives, and regulatory challenges for industrial application. Frontiers in Bioengineering and Biotechnology. 2020 Sep 3;8:914. [Crossref], [Google Scholar], [Publisher]
- Azarpour A, Zendehboudi S, Mohammadzadeh O, Rajabzadeh AR, Chatzis I. A review on microalgal biomass and biodiesel production through Co-cultivation strategy. Energy Conversion and Management. 2022 Sep 1;267:115757. [Crossref], [Google Scholar], [Publisher]
- Samuel HS, Etim EE, Shinggu JP, Bako B. Machine learning of Rotational spectra analysis in interstellar medium. Communication in Physical Sciences. 2023 Nov 25;10(I). [Google Scholar], [Publisher]
- Benedetti M, Vecchi V, Barera S, Dall’Osto L. Biomass from microalgae: the potential of domestication towards sustainable biofactories. Microbial Cell Factories. 2018 Dec;17:1-8. [Crossref], [Google Scholar], [Publisher]
- Chu WL. Strategies to enhance production of microalgal biomass and lipids for biofuel feedstock. European Journal of Phycology. 2017 Oct 2;52(4):419-37. [Crossref], [Google Scholar], [Publisher]
- Benedetti M, Vecchi V, Barera S, Dall’Osto L. Biomass from microalgae: the potential of domestication towards sustainable biofactories. Microbial Cell Factories. 2018 Dec;17:1-8. [Crossref], [Google Scholar], [Publisher]
- Tan CY, Dodd IC, Chen JE, Phang SM, Chin CF, Yow YY, Ratnayeke S. Regulation of algal and cyanobacterial auxin production, physiology, and application in agriculture: an overview. Journal of Applied Phycology. 2021 Oct;33(5):2995-3023. [Crossref], [Google Scholar], [Publisher]
- Shen XF, Xu YP, Jiang YF, Gao LJ, Tong XQ, Gong J, Yang YF, Zeng RJ. Evaluating nutrient limitation in co-culture of Chlorella pyrenoidosa and Rhodobacter sphaeroides. Science of The Total Environment. 2024 Jan 1;906:167706. [Crossref], [Google Scholar], [Publisher]
- Nie X, Mubashar M, Zhang S, Qin Y, Zhang X. Current progress, challenges and perspectives in microalgae-based nutrient removal for aquaculture waste: A comprehensive review. Journal of Cleaner Production. 2020 Dec 20;277:124209. [Crossref], [Google Scholar], [Publisher]
- Zhang B, Li W, Guo Y, Zhang Z, Shi W, Cui F, Lens PN, Tay JH. Microalgal-bacterial consortia: from interspecies interactions to biotechnological applications. Renewable and Sustainable Energy Reviews. 2020 Feb 1;118:109563. [Crossref], [Google Scholar], [Publisher]
- Nassema Rasheed R, Pourbakhtiar A, Mehdizadeh Allaf M, Baharlooeian M, Rafiei N, Alishah Aratboni H, Morones-Ramirez JR, Winck FV. Microalgal co-cultivation-recent methods, trends in omic-studies, applications, and future challenges. Frontiers in Bioengineering and Biotechnology. 2023 Sep;11:1193424. [Crossref], [Google Scholar], [Publisher]
- Magdouli S, Brar SK, Blais JF. Co-culture for lipid production: advances and challenges. Biomass and Bioenergy. 2016 Sep 1;92:20-30. [Crossref], [Google Scholar], [Publisher]
- Das PK, Rani J, Rawat S, Kumar S. Microalgal co-cultivation for biofuel production and bioremediation: current status and benefits. BioEnergy Research. 2022 Mar;15(1):1-26. [Crossref], [Google Scholar], [Publisher]
- Fernández-Luqueño F, López-Valdez F, Medina-Pérez G, editors. Bio and nanoremediation of hazardous environmental pollutants. CRC Press; 2023. [Crossref], [Google Scholar], [Publisher]
- Mulbry W, Kondrad S, Pizarro C, Kebede-Westhead E. Treatment of dairy manure effluent using freshwater algae: algal productivity and recovery of manure nutrients using pilot-scale algal turf scrubbers. Bioresource technology. 2008 Nov 1;99(17):8137-42. [Crossref], [Google Scholar], [Publisher]
- Gupta RK, Gangoliya SS, Singh NK. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. Journal of food science and technology. 2015 Feb;52:676-84. [Crossref], [Google Scholar], [Publisher]
- Mujtaba G, Lee K. Advanced treatment of wastewater using symbiotic co-culture of microalgae and bacteria. Applied Chemistry for Engineering. 2016;27(1):1-9. [Google Scholar], [PDF]
- Chisti Y. Biodiesel from microalgae. Biotechnology advances. 2007 May 1;25(3):294-306. [Crossref], [Google Scholar], [Publisher]
- Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: a review. Renewable and sustainable energy reviews. 2010 Jan 1;14(1):217-32. [Crossref], [Google Scholar], [Publisher]
- Ullah K, Ahmad M, Sharma VK, Lu P, Harvey A, Zafar M, Sultana S, Anyanwu CN. Algal biomass as a global source of transport fuels: Overview and development perspectives. Progress in Natural Science: Materials International. 2014 Aug 1;24(4):329-39. [Crossref], [Google Scholar], [Publisher]
- Pereira H, Barreira L, Mozes A, Florindo C, Polo C, Duarte CV, Custódio L, Varela J. Microplate-based high throughput screening procedure for the isolation of lipid-rich marine microalgae. Biotechnology for biofuels. 2011 Dec;4:1-2. [Crossref], [Google Scholar], [Publisher]
- Droop MR. Vitamins, phytoplankton and bacteria: symbiosis or scavenging?. Journal of plankton research. 2007 Jan 1;29(2):107-13. [Crossref], [Google Scholar], [Publisher]
- Casanova LM, Mendes LB, Corrêa TD, da Silva RB, Joao RR, Macrae A, Vermelho AB. Development of microalgae biodiesel: Current status and perspectives. Microorganisms. 2022 Dec 22;11(1):34. [Crossref], [Google Scholar], [Publisher]
- Satpati GG, Dikshit PK, Mal N, Pal R, Sherpa KC, Rajak RC, Rather SU, Raghunathan S, Davoodbasha M. A state of the art review on the co-cultivation of microalgae-fungi in wastewater for biofuel production. Science of The Total Environment. 2023 Apr 20;870:161828. [Crossref], [Google Scholar], [Publisher]
- Nassema Rasheed R, Pourbakhtiar A, Mehdizadeh Allaf M, Baharlooeian M, Rafiei N, Alishah Aratboni H, Morones-Ramirez JR, Winck FV. Microalgal co-cultivation-recent methods, trends in omic-studies, applications, and future challenges. Frontiers in Bioengineering and Biotechnology. 2023 Sep;11:1193424. [Crossref], [Google Scholar], [Publisher]
- Rojas-Villalta D, Gómez-Espinoza O, Murillo-Vega F, Villalta-Romero F, Guerrero M, Guillén-Watson R, Núñez-Montero K. Insights into Co-Cultivation of Photosynthetic Microorganisms for Novel Molecule Discovery and Enhanced Production of Specialized Metabolites. Fermentation. 2023 Oct 30;9(11):941. [Crossref], [Google Scholar], [Publisher]